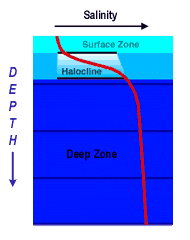
Salinity versus Depth
Salinity is a measure of dissolved salts in sea water. It is calculated as the amount of salt (in grams) dissolved in 1,000 grams (1 kilogram) of seawater. In much of Earth's oceans, there is a marked difference in salinity between the Surface Zone and the Deep Zone. An increase in salinity with depth is shown in red at left (<<<).
Although salinity generally increases with depth (<<<), there is a distinct layer where salinity increases sharply called the Halocline.
The electrical conductivity of seawater is strongly dependent on its salinity. Thus oceanographers use a "CTD" instrument to measure Conductivity, Temperature and Depth. From conductivity, they can calculate seawater salinity.


Hands on activities (include Standards & Benchmarks):
Stratified versus Mixed conditions
With no external forces applied, the ocean would stratify into a simple layered structure based on its density (mass divided by volume). Factors that influence seawater's density are:
- Pressure (which is related to water depth);
- Temperature; and
- Salt content (or salinity).
Sea Surface Salinity
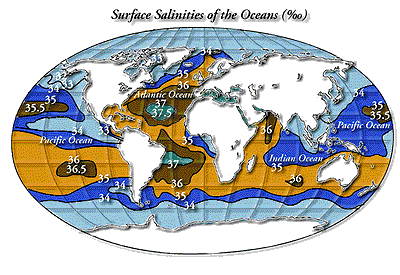
Salinity varies throughout the oceans, depending on whether freshwater (salinity = 0) has been added by precipitation or removed by evaporation (VVV).
Many regions are affected by processes that both increase and decrease salinity. For example, near the margins of continents, dry winds blow off landmasses and increase salinity via high evaporation. In these same areas, salinity is decreased by the influx of freshwater from nearby rivers. In general, the higher ocean salinities occur in the centers of ocean basins, where trade winds evaporate water and rain is rare. Lower salinities occur at high latitudes where the ocean receives fresh water from rain and melting ice.